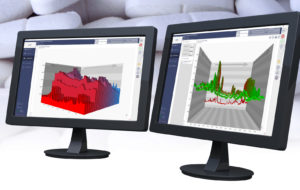
SynTQ V5.0 receives positive reception at INTERPHEX 2016
Manufacturing automation specialist Optimal Industrial Automation Ltd has marked its attendance at INTERPHEX 2016 by reporting great interest in its latest synTQ V5.0 process analytical technology (PAT) software platform. Offering faster development, coupled with a reduced time to market for batch production, plus, enabling the changeover to continuous manufacture and real time release for pharmaceutical products; the benefits offered by synTQ V5.0 were well received by exhibition attendees.
Supported by the Parenteral Drug Association (PDA), INTERPHEX is one of the largest pharmaceutical, biotechnology, manufacturing and medical device development events in the world. This year’s show enjoyed record attendance with attendees eager to engage with the latest developments in the industry. Held from the 26th-28th April at the Jacob K. Javits Center in New York – Optimal chose the exhibition to showcase the wide benefits of synTQ V5.0.
One of the key points of discussion during the show was drug shortages and the impact on patients. The production of pharmaceuticals is obviously highly regulated, so traditionally lead times for certain drugs can be high due to required testing throughout the process. However, synTQ V5.0 utilises process analytical technology to manufacture and evaluate pharmaceutical compounds continuously in real time to regulatory standards. A wide array of global pharmaceutical majors already using synTQ have reported reduced manufacturing times (sometimes from weeks to minutes) on production runs. Consequently, time to market for pharmaceuticals can be greatly reduced, increasing supply for the benefit of patients.
Martin Gadsby, Director of Optimal Industrial Automation commented subsequently, ‘there was a great deal of interest in synTQ V5.0 from potential clients and existing users of synTQ V4, for whom we have produced a clear product upgrade migration path. We received enquiries from laboratory teams, pilot plants and manufacturing users. Interest covered applications such as large and small molecule, primary and fill finish plus batch and continuous production methods. We enjoyed consistent interest during the 3 days of the exhibition – with many businesses enthusiastic with regards to becoming synTQ Application Partners.’
You can discover how synTQ can slash development and manufacturing times for pharmaceuticals by visitingwww.syntq.com.
Photo Caption 1: Optimal Industrial Automation Ltd has marked its attendance at INTERPHEX 2016 by reporting great interest in its latest synTQ V5.0 process analytical technology (PAT) software platform.
Photo Caption 2: synTQ V5.0 utilises process analytical technology to manufacture and evaluate pharmaceutical compounds continuously in real time to regulatory standards.
About Optimal Industrial Automation Ltd
Optimal has more than 25 years experience in the automation and optimization of manufacturing systems for the food, chemical, pharmaceutical and life science industries.
The demands being placed on manufacturers in relation to production costs, product quality and business sustainability are ever increasing; hence, the company’s primary aim is to deliver measurable improvements in all these target areas.
Optimal employs a wide-ranging skill set covering automation technologies, manufacturing processes and industry experience in highly regulated environments. As part of the implementation of these services Optimal has also developed a world leading PAT Data Management software package – synTQ® and the widely used integrated Print and Inspect system – synTI®.
The image(s) distributed with this press release may only be used to accompany this copy, and are subject to copyright. Please contact DMA Europa if you wish to license the image for further use.